
臺灣化學教育
Chemistry Education in Taiwan高瞻計畫教師參加ICCE 2016的經過和心得
劉曉倩1, *、蔡孟祐2
1, 2國立彰化高級中學
1教育部高中化學學科中心
*torrina01092002@yahoo.com.tw
n 參加會議經過
出席第24屆國際化學教育研討會(24th International Conference on Chemistry Education, ICCE 2016)是個偶然的機會,這幾年永續化學概念受到國際學者的重視,期許藉由教育促成及建立永續綠色化學發展,筆者與蔡孟祐老師在去年指導學生的科展作品「重金屬離子吸附暨檢測之循環系統」中就是以淨化水質及環保概念出發,ICCE國際教育化學研討會報名期間受到臺灣師範大學科學教育研究所邱美虹教授的鼓勵,第一次參與此國際盛會,內心實為惶恐,畢竟參加會議的成員多為國際知名教授,當筆者論文投稿被大會接受後,內心五味雜陳,因為會議的大多數演說內容有很多教育的專有名詞,筆者本非科學教育出身,對於演講內容中的教學專業領域感到有壓力感,但是學科中心有一群熱血的高中教師夥伴同是參與此一盛會,即使再難的挑戰都有辦法一起去達成,內心頓覺踏實並不孤單!
第一天正式會議是由根岸英一(Ei-ichi Negishi)開場主講,根岸教授是日本化學家,普渡大學講座教授、美國文理科學院院士、美國國家科學院外籍院士。根岸教授是根岸反應(Negishi coupling)的發現者,在有機化學界享有盛譽,因為在「有機合成中的鈀催化交叉偶聯反應」方面做出貢獻,而與理察·赫克(Richard F. Heck)、鈴木章(Akira Suzuki)共同獲得2010年諾貝爾化學獎,最特別的是他的出生地不在日本,也是唯一在滿洲國領土出生的諾貝爾獎得主。
根岸教授的演說以自己的論文研究為主,難度很高,簡報內容好多反應式,電子在幾個反應機構中跳躍,金屬催化有機化學反應基本上就是很環保的作法,因為金屬可以重複使用且對環境不會造成傷害,無奈的是儘管筆者對有機化學的反應機構推論雖然十分欣賞,但是純化學的演講對從事高中教育多年的教師障礙不小,一場演講下來,多數回憶起求學時對有機化學的迷戀。反思在高中課程中,有機化學被排定在選修化學下,學生適逢學測結束,對於下學期課程的熱情不在,課綱又將有機化學內容不斷刪減,教師也無需在解說反應機構上多所著墨,幾年下來功力倒退不少,大師的電子解說留下遺憾,平白辜負根岸教授一片苦心。
第二場演講是由著名英國化學家Prof. Peter Atkins(見圖一)主講,教授曾任牛津大學林肯學院的研究員與講師,研究專長為量子理論,主要教授量子力學與量子化學。他除了研究與教學外,勤於筆耕,在專業教科書方面,Atkins教授寫的〈物理化學〉、〈無機化學〉及〈分子量子力學〉是目前史上最暢銷的三本化學教科書。他對科普教育也十分熱衷,將化學以沒有門檻的方式介紹給一般讀者,著作約有二十餘本,其中翻成中文版的有〈化學元素王國之旅〉和〈化學分子世界導覽〉。
Atkins教授演說簡報善用圖表呈現,清楚易懂的文字敘述,精采動人的圖片,包括史料、畫作、歷史照片、科學攝影等,展現教授在藝術與科學之間的功力,讓聽者很快進入教授的元素世界,獲得很大的啟發。Akins教授演說幽默生動,回答問題時甚至有些俏皮。教授的科普寫作更善於將科學轉化成故事般趣味的閱讀,筆者想起Atkins教授曾經說過的一段話「當你打開這本書的時候(化學元素王國之旅),我希望你也能加入這想像之旅,一起探索方度謹嚴的導航圖:元素週期表。只不過在我們眼中它會是個虛擬國家,名為「週期王國」,這個生意盎然的國度,有丘陵、山脈、峽谷以及平原,我們在廣闊的草原散步,漸漸地會發現蘊藏於地底的隱藏結構,一種未知的運作原則,控制及統御整個王國!
圖一:與國際知名教授Prof. Peter Atkins(右二)合影
n 與會心得
神奇的奈米磁性流體是由奈米磁顆粒、界面活性劑以及承載溶劑所組成的,合成方式目前在網路幾乎都可以找的到,筆者與蔡孟祐老師發表的論文是利用共沉澱法,將氯化亞鐵(或硫酸亞鐵)與氯化鐵依1 : 2的比例混合後,加入過量的氨水,製備奈米級的四氧化三鐵顆粒,利用聚乙烯醇與硼砂產生凝膠化,吸附奈米磁微粒,過濾水中的重金屬離子(發表論文摘要和全文見附錄)。因為實驗裝置及奈米磁微粒複合聚乙烯醇材料可以重複使用,對於教導學生「綠色化學」,降低環境汙染及環保回收再生,有很大的助益。
張貼海報發表時,一位瓜地馬拉教授(見圖二)對於磁性流體很感興趣,當奈米磁顆粒加入水中時,它們會因凡得瓦力的吸引,凝聚成較大、較重的團塊體,因而無法藉由布朗運動而懸浮在水中,所以有其他教授建議可深入探討包覆磁性顆粒的界面活性劑種類,避免凝聚效應發生。
圖二:筆者與瓜地馬拉教授(左一)討論發表論文合影
一位教授也指出一般適用界面活性劑通常可分成兩大類,一是具親水端、親油端型的,如油酸;另一種是具陰、陽離子型的,如氫氧化四甲銨。油酸會利用親水端向內包圍奈米磁顆粒,長鏈狀的親油端向外,形成油膩狀的包覆層,油膩狀的包覆層會拉開奈米顆粒間的距離,避免凝聚現象的發生。而氫氧化四甲銨是四甲基銨離子(陽離子)和氫氧根(陰離子)所組成,氫氧根離子先接近磁性顆粒,而四甲銨離子會因電性相吸,包覆在最外圍,成為帶正電的膠體,膠體間因庫倫排斥力而穩定懸浮在水中,藉由改良奈米磁微粒可以進一步改良水中重金屬離子吸附效果,整個研究成果會更完整。
一般而言,磁流體的應用主要於電子業、機械業等精密儀器上,如馬達的旋轉軸承的真空軸封。磁流體對旋轉軸不產生機械性的摩擦,具有低磨損、無碎屑汙染、高速低滯等優點,廣泛地應用在自動化機械手臂製造上。在醫學領域上,鐵磁流體可被用於癌症檢測以及抗癌的臨床實驗上。像筆者將奈米磁微粒複合材料運用在吸附水中重金屬離子的研究報告並不多,教授們均十分肯定筆者團隊研究成果!
n 建議
古晉(Kuching)位於婆羅洲砂勞越州西端砂勞越河(Sarawak River)河畔,是馬來西亞砂勞越州的首府,而砂勞越州位於馬來西亞半島的東邊,所以有東馬之稱。因古晉馬來語的發音跟「貓」很相近,故又稱「貓城」,當地人也將錯就錯以貓作為城市的象徵,市內建有好幾座貓雕塑,甚至還設有一間以貓為主題的貓博物館,饒富趣味。(見圖三)
圖三:筆者與古晉市內著名貓雕塑合影
雖然古晉是砂勞越最大城市和馬來西亞第四大城市,但穿梭其中卻沒有大城市的繁囂,黃昏時漫步在古晉河畔(Kuching Waterfront)的沿河步道上,老巴剎帶著濃濃的英式建築風情,搭著小舢舨來回砂勞越河兩岸,更是悠閒寫意的賞心樂事。
古晉原屬於汶萊,1841年由英籍詹姆士.布魯克(James Brooke)乘著當地內亂的局勢,成為砂勞越的統治者,為砂勞越創造了歷史繁榮的「白酋長時代」,古晉目前仍遺有不少英國殖民地時代的建築物。但古晉的華人也不少,因此語言方面也是多姿多彩的,馬來語、英語與華語被當地的居民廣泛地使用。
大馬以伊斯蘭教為國教,但仍屬於宗教自由的國度,本地華人多信奉佛教、道教、基督教、天主教等等,所以當地的各式宗廟及建築十分多元,連食物也與西馬明顯不同,古晉勒沙及各式甜點結合當地熱帶植物素材,口味十分獨特。開會期間深刻感受到大會工作團隊對開會現場佈置的用心,連中場休息的小餐點都帶有濃濃的古晉風情,切成條狀後的千層糕像極了積木,外觀十分引人注目,雞蛋、牛奶及香醇的奶油風味,口感像極了臺灣的阿默蛋糕,很受歡迎!(見圖四)
圖四:古晉知名甜點千層蛋糕
唯一遺憾的是,馬國在處理ICCE報名細節上常有疏漏之處,譬如筆者在四月中即開始網路報名參與ICCE會議事宜,但是大會回函確認信通常都要一~二個星期,且刷卡報名流程設計上出現小錯誤,造成報名者可能重複刷卡的情況。去函詢問報名細節時,回函都要一星期以上,當然可能是國際會議報名人數較多,工作人員較不足所致,希望日後若有國際會議活動時,在網路報名流程架設上可以多留意些,避免一些小困擾。
整體而言,整個ICCE會議氣氛溫馨,即使是國際知名教授無論是在白天開會或是晚宴現場都十分親切,能與Prof. Robert (Bob) Bucat(見圖五)一起討論化學教育是此行一大收穫,參與化學教育年會瞬間擴展了自己的視野,雖然開會現場中有些演講太多專業領域有時無法融入,但是經過幾次會議下來也漸能以開闊的心去接受。教育下一代雖沒有一定的教學模式,但是啟發孩子的好奇心,激發學習潛能絕對是必要的,每個國家的教學設備不同,經費也各有差異,但身為教學第一線的教師,應該充滿熱情並會善用週遭生活的器材啟發學生。開會期間能與Lida Schoen教授一起參與分子官能基桌遊教學活動是此行另一大收穫,Lida教授雖然已經七十幾歲,但在化學教育上仍充滿熱情,這次與會期間她帶領臺灣教師到馬國當地學校教導化學在化妝品上的應用—髮膠、沐浴鹽、洗髮精及乳液工作坊,成功吸引很多國際學者的目光。
圖五:筆者與Prof.Robert(Bob)Bucat合影
n 感謝
感謝行政院科技部第二期高瞻計畫贊助此次赴古晉參訪國際化學教育年會(ICCE)所需之機票及住宿生活費用,以及對本校高瞻計畫的支持和指導。感謝國立彰化高級中學行政團隊和高瞻助理石景文先生承辦整個相關業務。最後更要感謝多日陪伴我們的臺灣師範大學邱美虹教授及全國化學學科中心教師團隊(見圖六),一起共同體驗這難得的日子。
圖六:教育部高中化學學科中心部分教師夥伴與其他國家教師交流合影
n 附錄:發表論文摘要和全文
I. Motivation
The Advanced Semiconductor Engineering dumped toxic wastewater in the Houjin creek in 2013. The pollution not only made the pH value of creek water reached 3.02 but also made the Nicole concentration up to 4.38 ppm. The polluted water also polluted the neighbor farmland. This pollution event aroused people’s conscious for rice food safety and made people understanding the important of clean water. If we have mobile water quality testing equipment, it can be used to do the quick testing for water quality and may alert the pollution. That is the reason why we develop this circulation system and establish a comparison curve of heavy metal ions concentration to measurement equipment for this system. By the adsorption effect of nano-iron-oxide for heavy metal ions, we make a simple filter which can effectively reduce the concentration of heavy metal ions. We also study how to achieve the optimum adsorption effect.
II. Research objects
1. To study a new type of nanomagnet-PVC composite.
2. To build a circulation system for adsorbing and detecting heavy metal ions.
3. To establish an analysis process of metal ion concentration by photoresistor.
4. To study the adsorption effect of composite under different conditions.
5. To enhance the adsorption effect of nanomagnet by changing the shape of filter.
6. To study how to increase the lifetime and efficiency of nanomagnet.
III. Experimental equipment
Equipment
|
Item |
Quantity |
|
Beaker (1000 ml) |
several |
|
Electronic scales |
1 |
|
Graduated pipet |
1 |
|
Stir bar and Hot plate stirrer |
1 |
|
pH meter |
1 |
|
Circulation system for adsorption and detection heavy metal ions |
1 |
Chemical
|
Ferric chloride |
Sodium acetate |
|
Ferrous sulfate |
2.9-Dimethyl-1.10-phenanthroline |
|
ammonia |
Sodium hydroxide |
|
Alcohol (95%) |
Copper sulfate |
|
1.5- diphenylcarbazide |
Solution of potassium dichromate |
|
acetone |
sulfuric acid |
|
Hydroxylamine hydrochloride |
Sodium citrate |
IV. Research principals and methods
1. Principals
(1)Nanomagnet
Natural magnetite is a kind of ferromagnetic mineral. When the magnetite turns into nanometer size, it will become superparamagnetic material. That is because when the size of mineral is nanometer level, both the physical and chemical properties will change. The smaller the particle size the larger the surface area, so the nano-particle can provide more space to adsorb heavy metal ions. Besides, the increase in the number of atoms by the surface then the surface energy also increased rapidly. Such that the surface atoms possess strong chemical activity and more easily combine with heavy metal ions.
(2) Nanocomposites
Nanocomposites are made by basing in resin, rubber, ceramics, and metal then combining with nano-particle. The manufacture process is dispersing the modifier in the matrix material uniformly to form a composite containing the nano-particle.
(3) Photoresistor
In bright condition, the resistance of photoresistor will decrease. In dim condition, the resistance of photoresistor will increase. For a certain wavelength of light, the resistance value will proportional to the concentration of heavy metal ions.
2. Experimental instrument
(1) Figure 1 shows the concentration measurement equipment for heavy metal ions. The light emitted from LED on the top will pass through the heavy metal solution in the tube then reach the photoresistor in the bottom. Use a multimeter to measure the resistance of photoresistor. By a comparison curve of resistance vs. concentration of heavy metal ions, we will know the concentration of heavy metal ions in the solution. We use black paper to wrap the instrument tightly in order to avoid light from outside affecting the accuracy of the experiment.
Fig. 1. The concentration measurement equipment for heavy metal ions
(2) Figure 2 shows the heavy metal ion adsorption filters. The black sponge in the lower left tube is the nanomagnet-PVC composite which will adsorb heavy metal ions when the solution passes through.
Fig. 2. The filter for heavy metal ion
(3) Figure 3 shows the whole circulation system for adsorbing and detecting heavy metal ions. The sample solution will be pumped from the beaker to the entire system. First, the solution will pass through the concentration measurement system. We chose the light color of LED and photoresistor depending on the color of sample solution. The light intensity will change after absorbing by the solution in the tube and the resistance of photoresistor will response to the light intensity. Next, the sample solution will pass through the filter and the nano-iron-oxide will adsorb the heavy metal ions. Finally, the sample solution will return to the beaker. Because the circulation system consists of several components, we can make any desired system as demand.
Fig. 3. The circulation system for adsorbing and detecting heavy metal ions
V. Experimental results
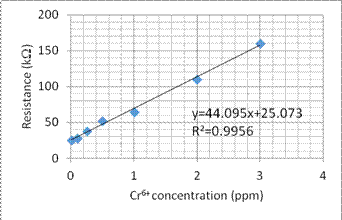
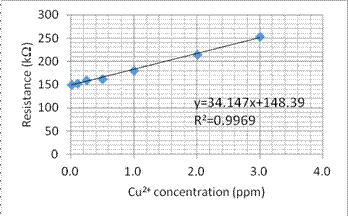
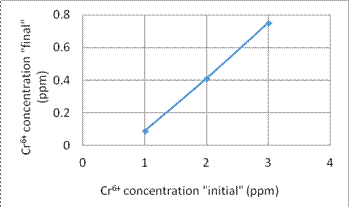
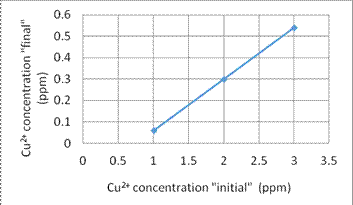
1. We use some different concentration of Cr6+ and Cu2+ solution to build the comparison curve of concentration to the resistance. Figures 4 and 5 show the comparison curve for Cr6+ and Cu2+ solution, respectively.
Fig. 4. The comparison curve for Cr6+ concentration
Fig. 5. The comparison curve for Cu2+ concentration
2. In the following we show the adsorption efficiency of nanomagnet composite under different conditions.
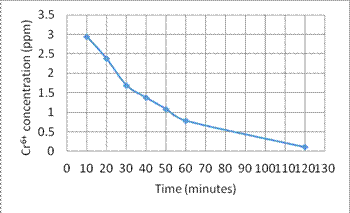
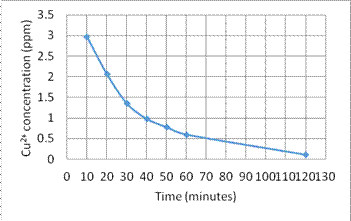
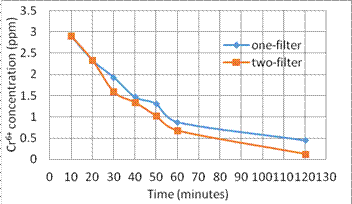
(1) Figures 6 and 7 show the circulation time period vs. the reduction of concentration of Cr6+ and Cu2+, respectively.
Fig. 6. The concentration reduction of Cr6+ vs. time
Fig. 7. The concentration reduction of Cu2+ vs. time
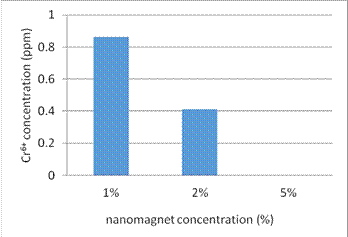
(2) Figure 8 shows the number of filters to the adsorption efficiency of Cr6+.
Fig. 8. The number of filters to the adsorption efficiency of Cr6+
(3) Figures 9 and 10 show the adsorption efficiency of filter for different initial concentration of Cr6 + and Cu2 + in 60 minutes respectively.
Fig. 9. The adsorption efficiency for different initial concentration of Cr6+
Fig. 10. The adsorption efficiency for different initial concentration of Cu2+
(4) Figure 11 shows the adsorption efficiency of Cr6+ for the different concentration of nanomagnet in PVC composite.
Fig. 11. The adsorption efficiency under different nanomagnet concentration
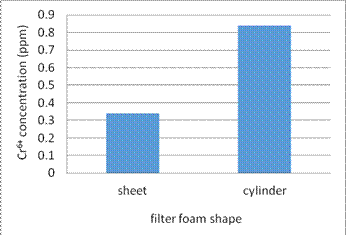
(5) Figure 12 shows how the filter foam shape affects the adsorption efficiency of Cr6+.
Fig. 12. Compare the adsorption efficiency of filter foam shape in sheet and cylinder
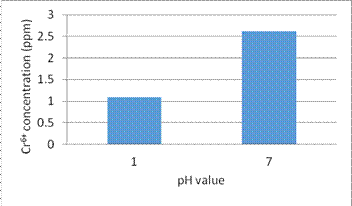
(6) We use the HCl solution in different pH value (1 and 7 respectively) to desorption heavy metal ions adsorbed in the filter for 30 minutes then use the desorbed filter to absorb Cr6+ for 60 minutes again. Figure 13 shows the desorption effect for different pH value.
Fig. 13. The desorption effect for different pH value solution
VI. Discussion
1. In the experiment (1), the result shows the nanomagnet and PVC composite can adsorb heavy metal ions effectively. The concentration of heavy metal ions reduce from 3 ppm to 0.11 ppm within 2 hours.
2. In the experiment (2), we study the effect of number of filters to the adsorption. When use two filters, owing to the more nanomagnets the concentration is lower after filtering in the same time period. But the more filters will slow down the flow rate of solution. The entire adsorption effect does not cause a great difference.
3. In the experiment (3), we study the adsorption efficiency under different initial concentration of Cr6+ and Cu2+. The results show that for the final concentrations, the results show an arithmetic progression for different initial concentrations after a certain filtration time. That means the adsorption rate is linearly.
4. In the experiment (4), we study adsorption efficiency of different concentration of nanomagnet in the PVC composite for Cr6+. The lower concentrations of nanomagnet due to the less number of nanoparticles make the adsorption efficiency lower, too. The higher concentration of nanomagnet makes the experiment result not available due to the more number of nanoparticles rushing into and contaminating the solution. It can exchange different composite materials to strengthen the adhesive force of nanomagnet to the material.
5. In the experiment (5), we use different filter foam shape to enhance the adsorption efficiency of Cr6 +. The results show if the filter foam shape is small piece of sheet, the adsorption efficiency is much better. This is because there is more surface area of small piece of sheet than cylinder shape and the heavy metal ions are more easily to contact the nanomagnet.
6. In the experiment (6), we study how to effectively recycle the used filter. The results show the lower pH value solution can desorb the heavy metal ions from nanomagnet effectively, and the desorbed nanomagnet and PVC composite filter can reuse again.










Please give us your valuable comment